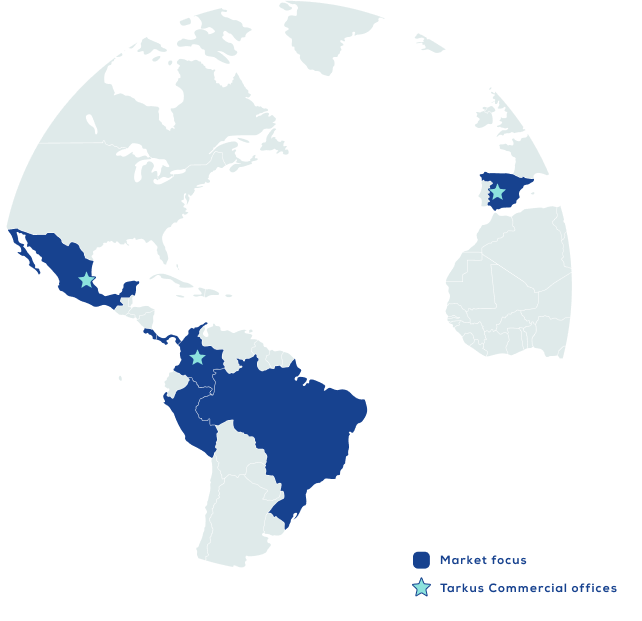
We operate under GMP certifications from INVIMA and ANVISA, ensuring safe and effective products tailored to the strict requirements of highly regulated markets like Brazil, Europe, and Australia.
We specialize in the production of active ingredients and finished products for highly regulated markets, focusing on chronic pain, anxiety, and neurological disorders. Our model combines clinical research, pharmaceutical development, and specialized manufacturing, ensuring that each product is backed by science and regulatory compliance.